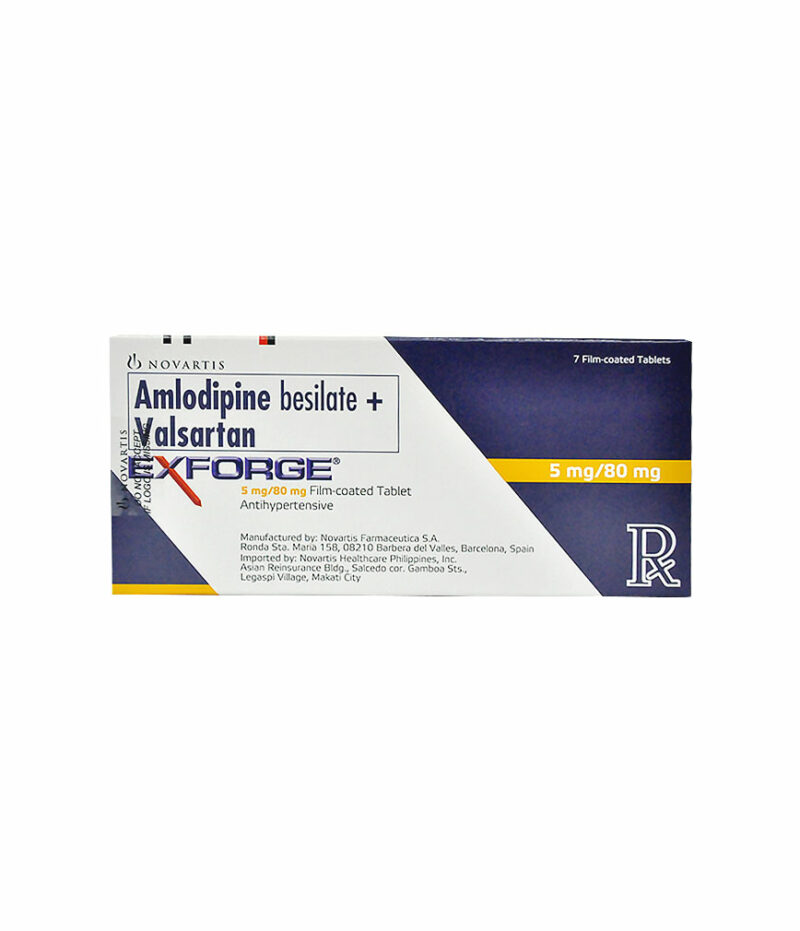
Exforge 5Mg/80Mg Tablet
₱50.00
IMPORTANT NOTICE: We require a doctor’s prescription for this product. Don’t forget to attach a copy of your valid prescription (.jpeg, .pdf, or .png format) upon checkout, or email it to [email protected] with your order number! To confirm your order and validate your prescription, our pharmacist will be in touch after you place your order. For a smoother transaction upon delivery, please be ready to present the original copy of your prescription when claiming your order.
* SOLD PER TABLET
Exforge 5Mg/80Mg Tablet
Generic Name: AMLODIPINE BESILATE 5MG, VALSARTAN 80MG
5 mg/80 mg: 5 mg of amlodipine (as amlodipine besilate) and 80 mg of valsartan.
5 mg/160 mg: 5 mg of amlodipine (as amlodipine besilate) and 160 mg of valsartan.
10 mg/160 mg: 10 mg of amlodipine (as amlodipine besilate) and 160 mg of valsartan.
Excipients/Inactive Ingredients: 5 mg/80 mg: Cellulose microcrystalline; crospovidone; silica, colloidal anhydrous; magnesium stearate; hypromellose, macrogol 4000, talc, titanium dioxide (E171), iron oxide, yellow (E172).
5 mg/160 mg: Cellulose microcrystalline; crospovidone; silica, colloidal anhydrous; magnesium stearate; hypromellose, macrogol 4000, talc, titanium dioxide (E171), iron oxide, yellow (E172).
10 mg/160 mg: Cellulose microcrystalline; crospovidone; silica, colloidal anhydrous, magnesium stearate, hypromellose, macrogol 4000, talc, titanium dioxide (E171), iron oxide, yellow (E172), iron oxide, red (E172).
- Applicable for Metro Cebu order
- Credit Card, GCash, Maya, Bank Transfer and COD payment available
- Daily Operations - 8:00 AM to 6:00 PM
- Order Cut-off - 3:00 PM
- Free Shipping for 1,499 and above orders (Visayas & Mindanao)
- For Order below 1,499 delivery fee of 99.00 (less than 1 kilo) will be applied
- For Order below 1,499 delivery fee of 199.00 (1 -3 kilo) will be applied
- Credit Card, GCash, Maya and Bank Transfer payment available
- Description
- Reviews (0)
Description
EXFORGE 5MG/80MG TABLET
Description : 5 mg/80 mg: 5 mg of amlodipine (as amlodipine besilate) and 80 mg of valsartan.
5 mg/160 mg: 5 mg of amlodipine (as amlodipine besilate) and 160 mg of valsartan.
10 mg/160 mg: 10 mg of amlodipine (as amlodipine besilate) and 160 mg of valsartan.
Excipients/Inactive Ingredients: 5 mg/80 mg: Cellulose microcrystalline; crospovidone; silica, colloidal anhydrous; magnesium stearate; hypromellose, macrogol 4000, talc, titanium dioxide (E171), iron oxide, yellow (E172).
5 mg/160 mg: Cellulose microcrystalline; crospovidone; silica, colloidal anhydrous; magnesium stearate; hypromellose, macrogol 4000, talc, titanium dioxide (E171), iron oxide, yellow (E172).
10 mg/160 mg: Cellulose microcrystalline; crospovidone; silica, colloidal anhydrous, magnesium stearate, hypromellose, macrogol 4000, talc, titanium dioxide (E171), iron oxide, yellow (E172), iron oxide, red (E172).
Indications / Uses : Treatment of essential hypertension.
Amlodipine + valsartan (Exforge) may be used as initial therapy in patients who are likely to need multiple drugs to achieve blood pressure goals. The choice of amlodipine + valsartan (Exforge) as initial therapy for hypertension should be based on an assessment of potential benefits and risks.
Administration : May be taken with or without food.
Contraindications : Known hypersensitivity to the amlodipine, valsartan or to any of the excipients; Pregnancy (see Use in Pregnancy & Lactation); Concomitant use of angiotensin receptor antagonists (ARBs) – including valsartan – or of angiotensin-converting enzyme inhibitors (ACEIs) with aliskiren in patients with Type 2 diabetes (see Interactions).
Special Precautions : Patients with Sodium- and/or Volume Depletion: Excessive hypotension was seen in 0.4% of patients with uncomplicated hypertension treated with amlodipine + valsartan (Exforge) in placebo-controlled studies. In patients with an activated renin-angiotensin system (such as volume- and/or salt-depleted patients receiving high doses of diuretics) who are receiving angiotensin receptor blockers, symptomatic hypotension may occur. Correction of this condition prior to administration of amlodipine + valsartan (Exforge) or close medical supervision at the start of treatment is recommended.
If hypotension occurs with amlodipine + valsartan (Exforge), the patient should be placed in the supine position and, if necessary, given an i.v. infusion of normal saline. Treatment can be continued once blood pressure has been stabilized.
Hyperkalemia: Concomitant use with potassium supplements, potassium sparing diuretics, salt substitutes containing potassium, or other drugs that may increase potassium levels (heparin, etc.) should be used with caution and with frequent monitoring of potassium.
Patients with Renal Artery Stenosis: Amlodipine + valsartan (Exforge) should be used with caution to treat hypertension in patients with unilateral or bilateral renal artery stenosis, stenosis to a solitary kidney since blood urea and serum creatinine may increase in such patients.
Patients with Renal Impairment: No data is available for severe cases (creatinine clearance <10 mL/min.) and caution is therefore advised. No dosage adjustment of amlodipine + valsartan (Exforge) is required for patients with mild to moderate renal impairment.
The use of ARBs – including valsartan – or of ACEIs with aliskiren should be avoided in patients with severe renal impairment (GFR <30 mL/min) (see Interactions).
Patients with Kidney Transplantation: To date there is no experience of the safe use of amlodipine + valsartan (Exforge) in patients who have had a recent kidney transplantation.
Patients with Hepatic Impairment: Valsartan is mostly eliminated unchanged via the bile whereas amlodipine is extensively metabolized by the liver. Particular caution should be exercised when administering amlodipine + valsartan (Exforge) to patients with hepatic impairment or biliary obstructive disorders (see Pharmacology under Actions).
Angioedema: Angioedema, including swelling of the larynx and glottis, causing airway obstruction and/or swelling of the face, lips, pharynx, and/or tongue has been reported in patients treated with valsartan; some of these patients previously experienced angioedema with other drugs including ACE inhibitors. Amlodipine + valsartan (Exforge) should be immediately discontinued in patients who develop angioedema, and should not be re-administered.
Patients with Heart Failure/Post-Myocardial Infarction: In general, calcium channel blockers including amlodipine should be used with caution in patients with serious congestive heart failure (New York Heart Association (NYHA) functional class III-IV).
In patients whose renal function may depend on the activity of the renin-angiotensin-aldosterone system (e.g. patients with severe congestive heart failure), treatment with angiotensin converting enzyme inhibitors or angiotensin receptor antagonists has been associated with oliguria and/or progressive azotemia, and in rare cases with acute renal failure and/or death. Evaluation of patients with heart failure or post-myocardial infarction should always include assessment of renal function.
Patients with Acute Myocardial Infarction: Worsening angina pectoris and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease.
Patients with Aortic and Mitral Valve Stenosis, Obstructive Hypertrophic Cardiomyopathy: As with all other vasodilators, special caution is required when using amlodipine in patients suffering from aortic or mitral stenosis, or obstructive hypertrophic cardiomyopathy.
Dual Blockade of the Renin-Angiotensin System (RAS): Caution is required while co-administering ARBs, including valsartan, with other agents blocking the RAS such as ACEIs or aliskiren (see Interactions).
Use In Pregnancy & Lactation
Pregnancy: As for any drug that also acts directly on the RAAS, amlodipine + valsartan (Exforge) must not be used during pregnancy (see Contraindications). Due to the mechanism of action of angiotensin II antagonists, a risk to the foetus cannot be excluded. Administration of angiotensin converting enzyme (ACE) inhibitors (a specific class of drugs acting on the renin-angiotensin-aldosterone system, RAAS) to pregnant women during the second and third trimesters has been reported to cause injury and death to the developing foetus. In addition, in retrospective data, first trimester use of ACE inhibitors has been associated with a potential risk of birth defects. There have been reports of spontaneous abortion, oligohydramnios and newborn renal dysfunction when pregnant women have inadvertently taken valsartan.
There are no adequate clinical data with amlodipine in pregnant women. Animal studies with amlodipine have shown reproductive toxicity at dose 8 times the maximum recommended dose of 10 mg (see Pharmacology: Toxicology: Non-Clinical Safety Data under Actions). The potential risk to humans is unknown.
If pregnancy is detected during therapy, amlodipine + valsartan (Exforge) must be discontinued as soon as possible (see Pharmacology: Toxicology: Non-Clinical Safety Data under Actions).
Breast-feeding: It is not known whether valsartan and/or amlodipine are excreted in human milk. Valsartan was excreted in the milk of lactating rats. It is therefore not advisable for women who are breast-feeding to use amlodipine + valsartan (Exforge).
Women of Child-bearing Potential: As for any drug that also acts directly on the RAAS, amlodipine + valsartan (Exforge) must not be used in women planning to become pregnant. Healthcare professionals prescribing any agents acting on the RAAS should counsel women of childbearing potential about the potential risk of these agents during pregnancy.
Fertility: There is no information on the effects of amlodipine or valsartan on human fertility. Studies in rats did not show any effects of amlodipine or valsartan on fertility (see Pharmacology: Toxicology: Non-Clinical Safety Data under Actions).
Be the first to review “Exforge 5Mg/80Mg Tablet”
You must be logged in to post a review.















Reviews
There are no reviews yet.