Description
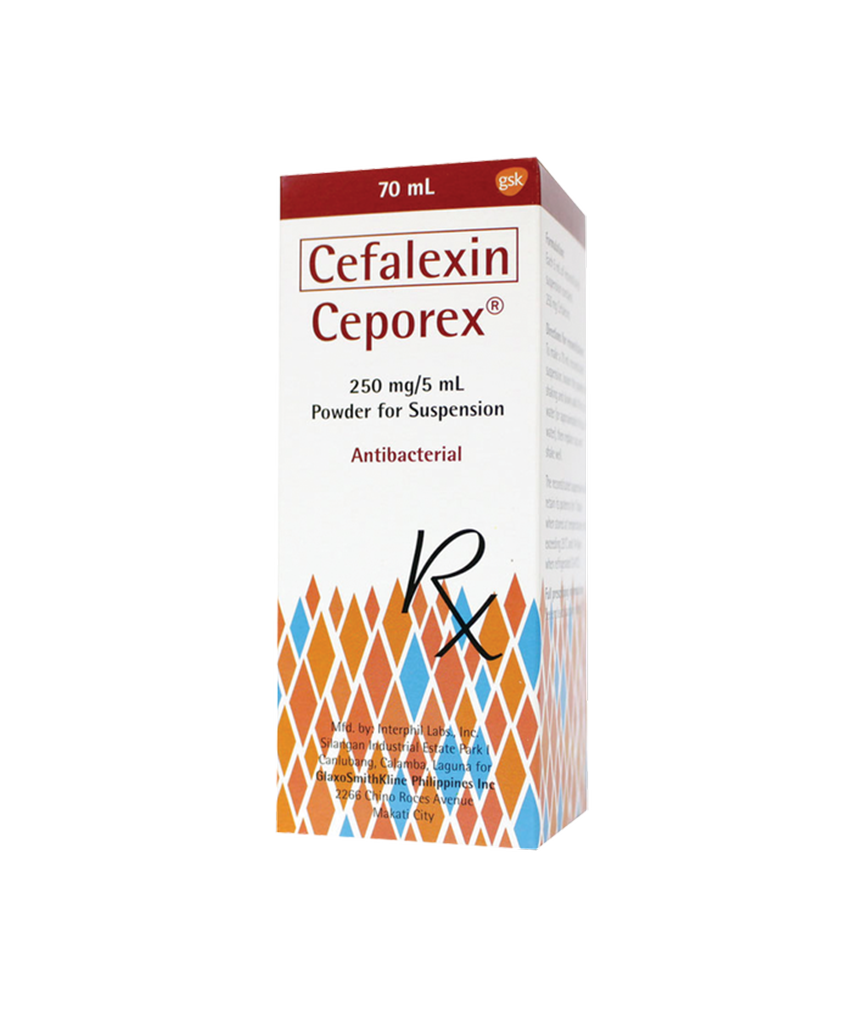
Ceporex Ds 250MG Suspension 70ML
Description :
Ceporex capsules contain cefalexin (as monohydrate) 250 mg or 500 mg. Ceporex capsules comply with the USP specifications for cefalexin capsules.
Ceporex suspension in both strength complies with USP specifications for cefalexin mixture.
Indications / Uses :
Ceporex is a bactericidal antibiotic which is active against a wide range of gram-positive and gram-negative organisms. It is indicated for treatment of the following conditions when caused by susceptible bacteria.
Treatment of respiratory tract infections (RTIs), urinary tract infections (UTIs), skin and soft tissue infections, otitis media and other infections due to sensitive organisms.
Administration :
May be taken with or without food: May be taken w/ meals to reduce GI discomfort.
Contraindications :
Hypersensitivity to the cephalosporin group of antibiotics.
Severe systemic infections, which require parenteral cephalosporin treatment, should not be treated orally during the acute stage.
Special Precautions :
Hypersensitivity Reactions: Cefalexin should be given cautiously to patients who have shown hypersensitivity to other drugs. Cephalosporins should be given with caution to penicillin-sensitive patients, as there is some evidence of partial cross-allergenicity between the penicillins and cephalosporins. Patients have had severe reactions (including anaphylaxis) to both drugs. If the patient experiences an allergenic reaction, cefalexin should be discontinued and treatment with the appropriate agents initiated.
Direct Coombs’ Test: Positive direct Coombs’ tests have been reported during treatment with cephalosporin antibiotics. For hematological studies, or in transfusion cross-matching procedures when antiglobulin tests are performed on the minor side, or in Coombs’ testing of newborns whose mothers have received cephalosporin antibiotics before parturition, it should be recognized that a positive Coombs’ test may be due to the drug.
False-Positive Glycosuria Reaction: A false-positive reaction for glucose in the urine may occur with Benedict’s or Fehling’s solutions or with copper sulphate test tablets. Tests based on glucose oxidation reactions may be safely used.
Renal Impairment: Cefalexin should be administered with caution in presence of markedly impaired renal function as it is excreted mainly by the kidneys. Careful clinical and laboratory studies should be made because the safe dosage may be lower than the usually recommended (see Dosage & Administration).
Sucrose: Ceporex suspension contains sucrose. Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take Ceporex.
Effects on the Ability to Drive and Operate Machinery: None reported.
Impairment of Fertility: There are no relevant data available.
Use in Pregnancy: There is no experimental or clinical evidence of teratogenic effects attributable to cefalexin, but Ceporex should be administered with caution during pregnancy.
Use in Lactation: Cefalexin is excreted in human milk in low concentrations and should be used with caution in nursing mothers.
Caution for Usage
Instructions for Use and Handling: Suspensions: Ceporex suspensions are prepared by adding water to the granules to give suspensions containing cefalexin 125 and 250 mg in each 5 mL.
Slowly add 60 mL of water. Replace cap and shake well.
Pediatric Oral Drops: Slowly add 6 mL of water. Replace cap and shake well. Discard cap and fit dropper. The dropper is calibrated at 0.25 mL, 0.5 mL and 1 mL.












Reviews
There are no reviews yet.